CPS Meeting 2019
Please find informations on our final project report meeting here:
Conference report:
PDF-File (1,7 mB)
Conference website:
www.cps2019.de
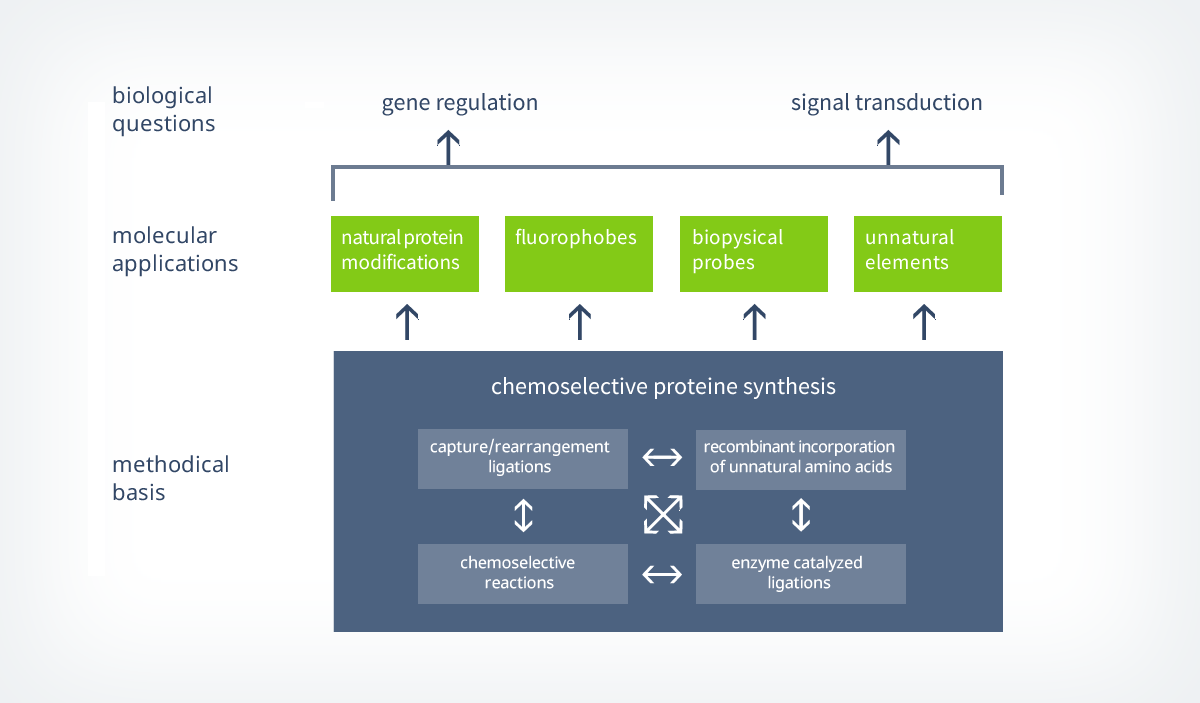
1.1. Strategies for the synthesis of functional proteins
Chemical ligation strategies for the synthesis of proteins are often based on a chemoselective capture-step, which is connecting to peptide fragments covalently, followed by an intramolecular rearrangement that is forming a native amide bond. This capture/rearrangement strategy is the foundation for one of the most often used ligation concepts in protein synthesis – the native chemical ligation (NCL).
In 1994, Kent et al. described the chemoselective coupling of an unprotected peptide-thioesters to a cysteine containing peptide for the first time and based on the research of T. Wieland.6 With this method, they were able to form a native amide bond next to a cysteine containing ligation site (scheme 2).7 First, a reversible transthioesterification step forms a thioester (capture), that is then further reacted to a intermolecular peptide bond by a S -> N shift (rearrangement). Within the last years, the NCL was further optimized and strategies were developed to enable ligations to peptides that do not contain cysteines next to the ligation site.
In addition to that, new capture-strategies based on the traceless Staudinger ligation were developed and novel synthesis routes towards peptidic thioesters were established. This broad repertoire of tools enables NCL to be used for convergent synthesis approaches in which multiple peptide fragments can be ligated selectively (e.g. shown by the kinetically controlled ligation).
As already mentioned before, NCL is not only a powerful tool for the ligation of several peptide fragments but also for the semi synthesis of whole proteins that exceed the size limit of classical peptide ligation strategies. Therefore, truncated proteins are recombinantly expressed and ligated to peptides that have been generated by solid phase peptide synthesis (scheme 2). With this in hand, NCL is an elegant and flexible way to site-specifically incorporate biophysical probes into a protein of choice.8 However, to ligate a recombinantly expressed protein to a synthetical thioester, the proteins needs to carry an N-terminal cysteine. This can typically be achieved by the combinational use of site-directed mutagenesis and controlled proteolytic cleavage.
Up to date, a variety of different proteins, like membrane proteins, cyclic proteins, proteins containing biophysical probes or cages and proteins containing posttranslational modifications (lypidation, phosphorylation, glycosylation) have been successfully synthesized by NCL or a similar technique called expressed protein ligation (EPL).3 EPL is used to recombinantly generate protein-thioesters that can be ligated to N-terminal cysteine containing peptides. In particular the recent syntheses of two glycosylated proteins, a homogenously glycosylated RNase9 and a prion-protein containing a GPI-anker,10 are impressive examples for the application of this powerful tool by german research teams.