CPS Meeting 2019
Please find informations on our final project report meeting here:
Conference report:
PDF-File (1,7 mB)
Conference website:
www.cps2019.de
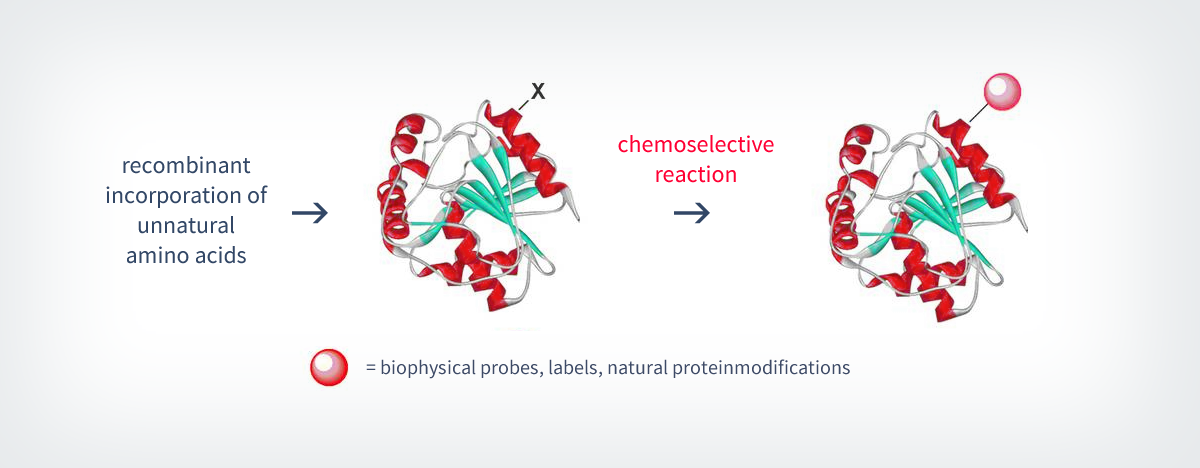
1.1.2. Recombinant incorporation of unnatural amino acids
The ligation methods described in chapter 1.1.1. enables scientists to synthesize homogenous natural proteins and to elegantly incorporate functional mojeties (e.g. protein modifications or probes) into the architecture of a protein.4,5 Proteins that have been generated by this strategy and therefore carry an unnatural functionality are addressed in subsequent chemoselective and/or bioorthogonal reactions11 and can thereby be conjugated to a functional moiety (scheme 3; further discussion on chemoselective reactions in chapter 1.1.3).12
In a recent application of this procedure, researchers were able to localize posttranslationally modified proteins within living organism. To do so, they coupled a bioorthogonal chemical reporter, in this particular case a biophysical probe, to an unnatural functionality within a biopolymer, that has been incorporated by the use of the cellular biosynthesis pathway of the cell.4
Up to date, there are several recombinant methods established for the incorporation of an unnatural moiety X (scheme 3). As already mentioned above, among them are methods to incorporate unnatural groups by the help of the natural cellular biosynthesis pathway. A very prominent example is the metabolic glycan engineering, which can be used to incorporate unnatural carbohydrates to glycoproteins.4a, 13
Genetic code engineering14 and genetic code expansion15 are two additional techniques of high importance for the SPP. Genetic code engineering enables the replacement of an amino acid of one type within the protein sequence using an auxotrophic expression system. In this case, site directed mutagenesis is not necessary. In contrast to that, genetic code expansion allows the site-directed incorporation of an unnatural amino acid and therefore replacement of a single unit within the protein sequence. This technique requires a genetically modified and adjusted translation machinery.16
Until now, both methods were successfully applied for the incorporation of a single unnatural amino acid into a protein of interest. However, very recently scientists were able to incorporate up to three different functional moieties/unnatural amino acids using these methods (separately or by a combinational approach).17 In addition to that, it was shown that it is possible to incorporate amino acids that already carry a posttranslational modification (acetyl lysine).18 These improvements enable scientists to combine several separate experiments into one single step.